
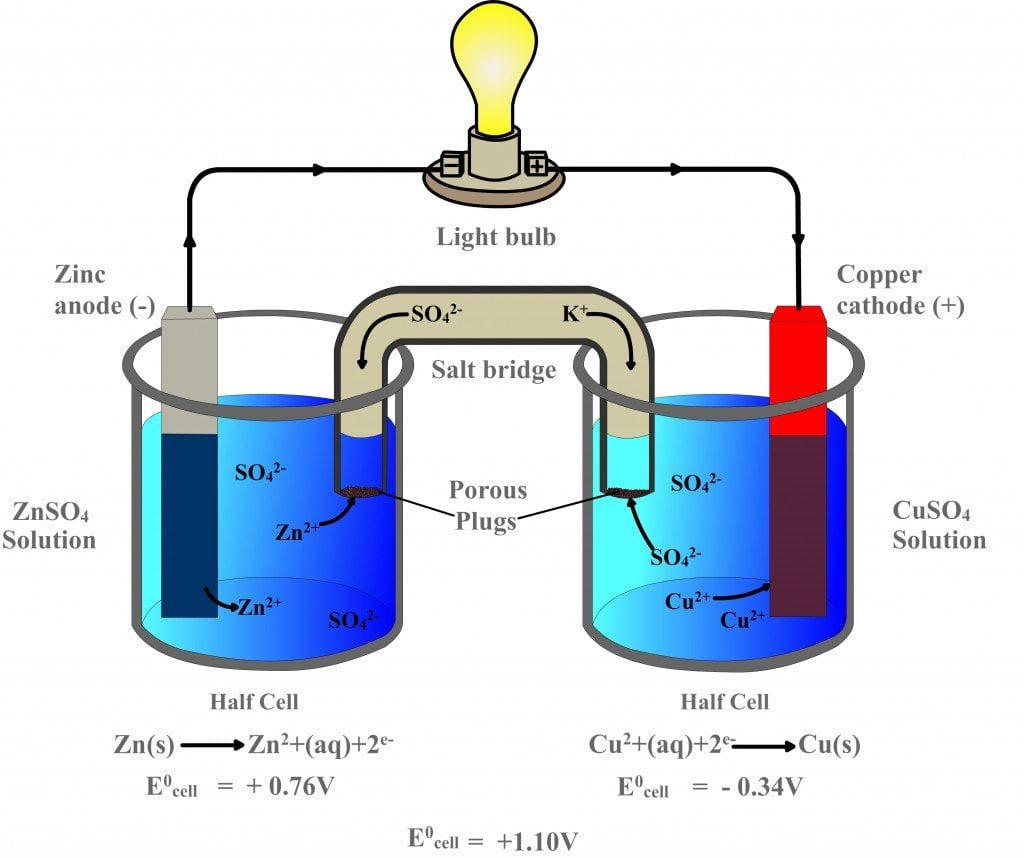
Ions being precipitated onto the copper electrode. At the same time, potassium ions from the salt bridge move into the copper half-cell in order to replace the Cu 2+ In the above wet-cell during discharge, nitrate anions in the salt bridge move into the zinc half-cell in order to balance the increase in Zn 2+ The salt bridge typically contains a high concentration of potassium nitrate (a salt that will not interfere chemically with the reaction in either half-cell). When the half cells are placed in two entirely different and separate containers, a salt bridge is often used to connect the two cells.
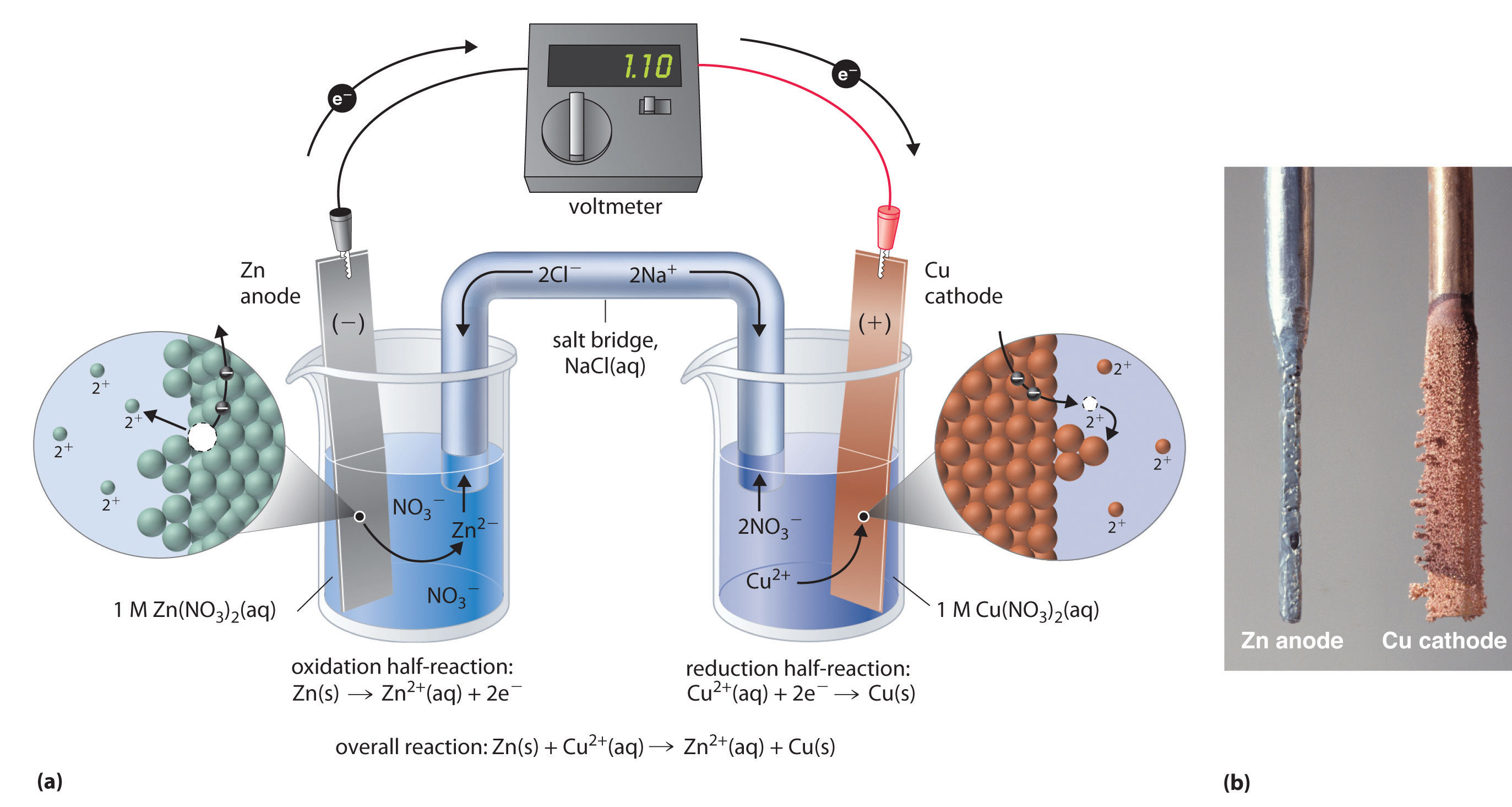
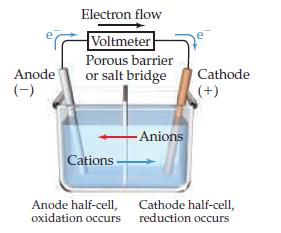
A porous barrier or ceramic disk may be used to separate the two solutions while allowing the flow of sulfate ions. Since neither half reaction will occur independently of the other, the two half cells must be connected in a way that will allow ions to move freely between them. This provides an electrical current that illuminates the bulb. Excess electrons produced by the oxidation of zinc metal are “pushed” out of the anode, which is therefore the negative electrode, travel through the wire and are "pulled" into the copper cathode where they are consumed by the reduction of copper ions. A wire and light bulb may connect the two electrodes.
The two half cells each support one half of the reactions described above. In classroom demonstrations, a form of the Daniell cell known as two half cells is often used due to its simplicity. At the anode (negative electrode), zinc is oxidized as per the following half reaction:ĭaniell cell demonstration made of zinc and copper electrodes in half cells In the Daniell cell, copper and zinc electrodes are immersed in a solution of copper(II) sulfate and zinc sulfate, respectively. With contemporary definitions, the standard potential of the Daniell cell at 25 ☌ is actually 1.10 V. The definitions of electrical units that were proposed at the 1881 International Conference of Electricians were designed so that the electromotive force of the Daniell cell would be about 1.0 volts. The Daniell cell is also the historical basis for the contemporary definition of the volt, which is the unit of electromotive force in the International System of Units. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the 1860s by a Frenchman named Callaud and became a popular choice for electrical telegraphy. The Daniell cell was a great improvement over the existing technology used in the early days of battery development. Zinc sulfate may be substituted for the sulfuric acid. He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile, and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Note: A light bulb is a example of a simple load where current (a flow of electrons) is used to resistively heat a filament of metal, usually tungsten, until it radiates energy in the form of visible light.īefore moving to the next page you should be able to recognize the parts of a simple voltaic cell.The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consists of a copper pot filled with a copper (II) sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode. Utilizes the flow of electrons to perform some function. The load is the part of the circuit which.The external circuit is used to conduct the flow of electrons between the electrodes of the voltaic cell and usually includes a load.The oxidation and reduction reactions are separated into compartments called half-cells.A salt bridge is a chamber of electrolytes necessary to complete the circuit in a voltaic cell.That uses a chemical reaction to produce electrical energy. Particles, either electrons or ions, through a conductor.Ī voltaic cell is an electrochemical cell And operation of a voltaic cell Working DefinitionsĮlectrical current is the movement of charged


 0 kommentar(er)
0 kommentar(er)
